Abstract
Background: Anemia is the most common cytopenia among patients with myelodysplastic syndromes (MDS) often necessitating regular red blood cell (RBC) transfusions. As a result, most patients develop iron overload (IO). RBC transfusion dependency and IO have been associated with worse clinical outcomes, including inferior overall survival (OS), in MDS patients. While iron chelation therapy (ICT) is recommended by most clinical guidelines and experts for select MDS patients, the use of ICT remains controversial in the absence of published, randomized controlled trials. To evaluate the impact of ICT on survival among lower risk MDS patients, we performed a systematic review and meta-analysis (SRMA) of the literature.
Methods: We conducted an SRMA according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines. PubMed and Embase electronic databases were searched through October 2016 with search terms "MDS" OR "myelodysplastic syndrome" AND "iron chelation" OR "iron chelating" OR "iron overload" AND "survival" OR "death" AND "study" OR "trial." Additionally, the Cochrane Library, the World Health Organization (WHO) International Clinical Trials Registry, and abstracts from recent American Society of Hematology annual meetings (years 2014-2016) were searched. One study, published in 2014, had an updated analysis in 2017 that was used for the current analysis. Information abstracted included data relating to study and patient characteristics, outcome measures (mortality and progression to acute myeloid leukemia [AML]), and measures of effect (calculated odds, hazard ratios [HRs], and confounding variables for adjustment). A Random Effects model was used to compute an adjusted HR (aHR) among the different studies. The Newcastle-Ottawa Scale (NOS) was used to assess the quality of studies. Study heterogeneity was assessed using the Cochran Q and the I2 statistic. Publication bias was assessed through visual examination of a funnel plot and with the Egger's test for small-study effects.
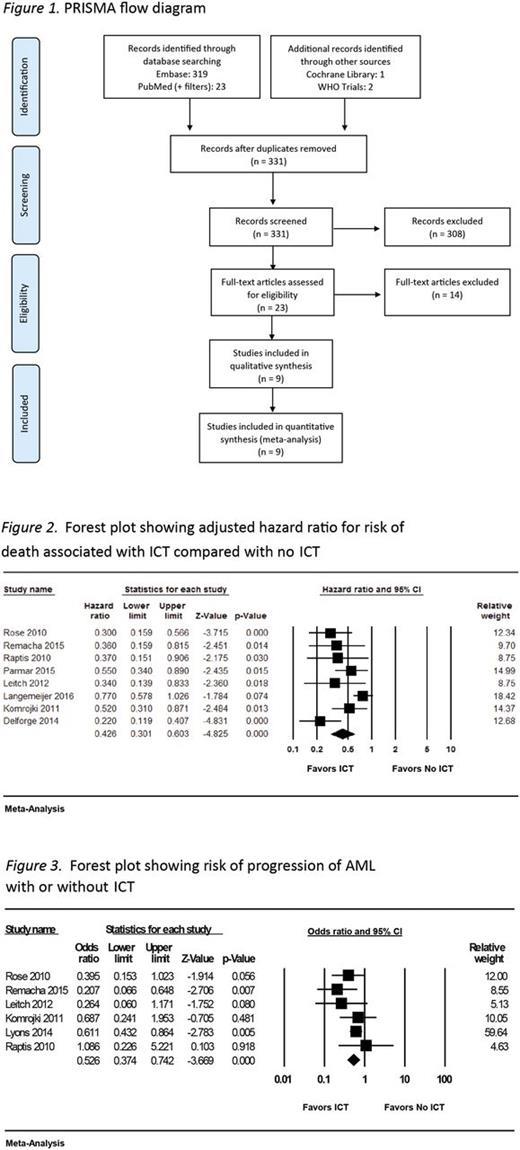
Results: Of the 331 records screened, 23 were assessed for eligibility, and 9 were included (Figure 1). Of these 9 studies, 6 were considered to be of high quality based on the NOS. All 9 were observational studies (4 prospective and 5 retrospective); 8 were identified as including patients with the International Prognostic Scoring System (IPSS)-defined low and intermediate-1 risk MDS. In these 8 studies, ICT was associated with an overall lower risk of mortality (aHR 0.43; 95% confidence interval [CI] 0.30-0.60; p < 0.01) (Figure 2). The pooled estimate of the ratio of median unadjusted survival times was 2.02 (95% CI 1.64-2.49), indicating that the median OS for patients receiving ICT was approximately twice that for patients who did not receive ICT. There was evidence of significant heterogeneity across studies for this outcome measure with I2 = 64% and Cochran's Q statistic = 19.8 (p = 0.006). Although the direction of effects did not change, heterogeneity was significant in prospective studies ( I2 = 73%; Cochran's Q statistic = 7.4, p = 0.02), but not in retrospective studies ( I2 = 9.8%; Cochran's Q statistic = 4.4, p = 0.35). In the 2 studies that reported on benefits of adequate chelation, any degree of chelation was found to confer a survival benefit, but this was to a lesser degree than the survival benefit accrued by those who received higher adequate doses. ICT seemed to decrease the risk of progression to AML (odds ratio 0.526; 95% CI 0.37-0.74; p < 0.001) in the studies that reported on this outcome (Figure 3); these studies displayed no significant heterogeneity. The impact of ICT on end organ damage could not be assessed due to non-uniform reporting of studies.
Conclusions: This SRMA suggests a significant reduction in the adjusted risk of mortality among IPSS low and intermediate-1 risk MDS patients treated with ICT. Furthermore, our analysis suggests that ICT is associated with delayed progression to AML, although the causality of these associations cannot be established based on this study. Randomized controlled studies are needed to confirm these findings.
Ballas: Novartis: Honoraria, Speakers Bureau. Zeidan: AbbVie, Otsuka, Pfizer, Gilead, Celgene, Ariad, Incyte: Consultancy, Honoraria; Takeda: Speakers Bureau; Otsuka: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal